Hydrogen peroxide is virtually the only bleaching agent available for protein fibers and it is also used very extensively for the cellulosic fibers. Hydrogen peroxide is a colorless liquid soluble in water in all proportions. It is reasonably stable when the pH is below 7, but tends to become unstable as the alkalinity increases. Commercial hydrogen peroxide, therefore, is made slightly acid so that it will not lose strength during storage. Solutions of hydrogen peroxide of more than 20 volumes cause intense irritation when they come into contact with skin and should be washed away immediately.
Cotton is usually bleached in 1-volume liquor at the boil. The most important factor in bleaching is to achieve the right degree of stability in the bleach liquor. If the pH were too low no per hydroxyl ions are set free and bleaching does not take place; when the liquor is too unstable the whole of the oxygen is liberated and escapes into the atmosphere before it has had time to act upon the cotton.
The bleaching liquor must be made alkaline, otherwise it would be too stable, but it is virtually impossible to adjust to the optimum pH with alkali alone and there is a marked tendency for the liquor to is too unstable, however carefully it has made alkaline. It is, therefore, necessary to add a stabilizer, and of all the substances, which have been, tried sodium silicate is the most effective.
Hydrogen peroxide is a stable chemical under acidic conditions and needs the addition of an alkali for activating it. Above pH 10, it is extremely unstable when it gets decomposed under water and oxygen.
This liberated oxygen, however, has no bleaching action and the catalysts are therefore a cause of loss of bleaching power. In fact, hydrogen peroxide is used bleaching under alkaline conditions (pH 10) after stabilizing at this pH by adding sodium silicate, borax, phosphate etc. Generally bleaching is done at 80ºC to 85ºC temperature.
Hydrogen peroxide solution at any concentration can be stable or unstable depending upon the several factors listed below.
1. pH: Stable in acidic solution and unstable in alkaline baths.
2. Temperature: As temperature increases the solution becomes increasingly unstable.
3. Buffers: Silicates, Phosphates, Borax, Proteins and others tend to stabilize peroxide.
4. Metals: (a) Ca and Mg in the presence of silicates tend to stabilize baths; (b) other metals, i.e., Cu, Fu, etc. tend to unstabilize bleach solutions.
5. Hard water: Depending upon the hardness of water and the metals making it hard, peroxide is unstabilize.
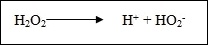
It was at one time believed that the bleaching action of hydrogen peroxide was due to the liberation of nascent oxygen but this explanation is no longer tenable. It is known that under certain conditions, particularly with regard to pH, hydrogen peroxide will liberate hydrogen and per hydroxyl ions in the following manner:
Hydrogen peroxide (H2O2) is a universal bleaching agent and is used extensively for the bleaching of cotton materials. The advantages in its use are:
1. It can be employed for bleaching fibers like wool, silk and jute also.
2. It requires less manipulation of fabric and hence less labor.
3. The loss in weight in bleaching is less than that with hypochlorite bleaching
4. Less water is required with peroxide bleaching and there is no need for souring after bleaching.
5. Peroxide bleached goods are more absorbent than hypochlorite bleached goods.
6. After – yellowing of white goods bleached with peroxide or less than with hypochlorite bleached goods.
7. Peroxide bleaching is safer in regard to chemical degradation and
8. Continuous scouring and bleaching in one operation is possible by employing peroxide.
Application Methods:
1- Batch or Discontinuous process:
e.g Kier, Jigger, Winch.
2- Continuous process:
e.g J-Box, Vaporloc
3- Semi-Continuous Process:
e.g Pad – Roll



Leave a comment